This blog post isn’t that cool due to the seriousness and harm it causes the ocean and the organisms in it, it is still a fact you didn’t ask for.
Ocean acidification is the ongoing decrease of the pH in the oceans, which is caused by the uptake of carbon dioxide from the atmosphere. pH is a measure of acidity or alkalinity. A pH below 7 is considered acidic, and a pH greater than 7 is considered alkaline, or basic.
Ocean acidification occurs due to the increase in human activity. The 1800 industrial revolution triggered the increase of carbon dioxide into the atmosphere which has then continued to rise ever since. The CO2 that is released into the atmosphere leads to what is known as atmospheric warming and climate change; around one third to a half of the CO2 that is released by human activities is absorbed into the oceans. Although this helps reduce the rate of CO2 that is being absorbed into the atmosphere, it has a serious direct and chemical effect on the seawater, which is why we call it ocean acidification.

The rise of the CO2 levels is caused by many factors, for example: the burning of fossil fuels, oil and gas, deforestation that results in the decrease in number of trees that are there to absorb the CO2 in the atmosphere, as well as when plants are cut down/burnt/left to rot which results in the carbon that makes up their organic tissue is released as CO2.
When carbon dioxide is absorbed by the seawater, chemical reactions take place that reduce the pH levels, carbonate ion concentration and saturation states of biologically important calcium carbonate minerals. Although photosynthetic algae and sea grasses may benefit from the higher CO2 conditions in the oceans, as they require CO2 to survive just like plants on land, studies have shown that the calcifying species, including oysters, clams, sea urchins, shallow water corals, deep sea corals and calcareous plankton are highly affected due to the acidity.
The organisms that produce calcium carbonate structures, such as skeletons and shells, have to use extra energy to either repairing their damaged shells or try thicken them to survive. The result of using this much energy could impact the organism’s ability to grow and reproduce. The organisms that are able to survive in such acidic waters are likely to become smaller which could potentially affect the food chain that relies on them. For example the pteropod (sea butterfly) are eaten by organisms ranging from krill to whales, as well as being a food source for North Pacific juvenile salmon.
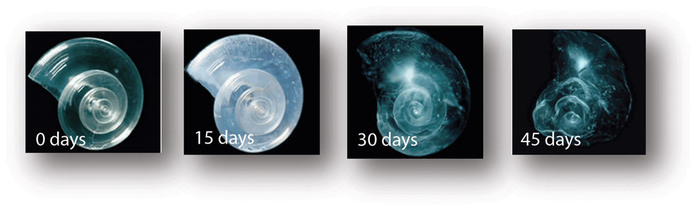
Corals are highly affected marine organisms, increasing ocean acidification has been shown to significantly reduce the ability of reef-building corals to produce their skeletons. Coral biologists reported that ocean acidification could compromise the successful fertilization, larval settlement and the ability to survive of the Elkhorn coral which is an endangered species. The results of this study suggests that ocean acidifcation can severely impact the ability of coral reefs to recover from this disturbance, as well as eroding faster than they can rebuild which can compromise the long-term viability of these ecosystems and impact the estimated 1,000,000 species that depend on the coral reef habitats.
